Case Study: Clinical Trial Project Support for Mid-sized Biotech.
At a Glance.
This was originally a US-based Biotech company that had expanded its operations to Europe. They had already established a team by the time i-Pharm started working with them.

The Challenge.
The team had eight permanent employees at the senior manager and director level in Clinical Operations, Regulatory Affairs, and Medical functions. Finding suitable candidates was challenging in some countries where they were new to the market. The permanent directors had experience in running trials in Europe, but it was difficult to find candidates with the necessary skill set for rare infectious diseases.
As a smaller Biotech company, they were struggling to attract permanent employees with the right skill set given the uncertain work beyond the next 18 months to two years.
The Solution.
i-Pharm collaborated with the Associate Director of Clinical Operations and suggested considering experienced contractors. This approach would reduce the number of required headcount as the contractors provided by i-Pharm possess substantial experience, enabling them to perform work in half the time.
The contractors had specific therapeutic area expertise and extensive experience with all external stakeholders and sites in Europe where the company intended to conduct trials. They could really help with strategically delivering the trials in Europe more quickly and effectively.
Therefore, the decision was made to take on contractors, and i-Pharm supported the build-out of that European team. Overall, the Biotech company needed seven Project Managers across Europe.
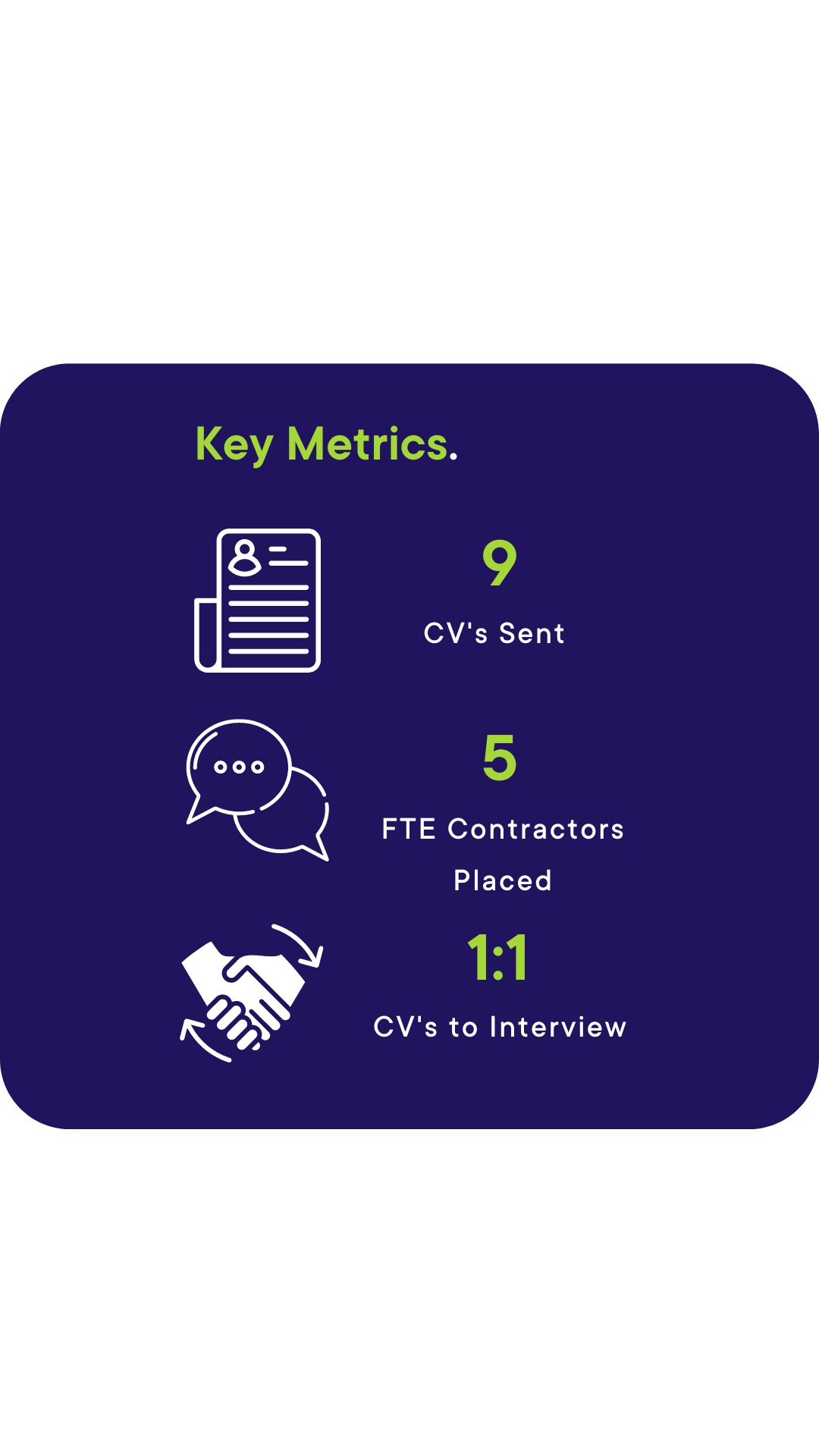
The Outcome.
i-Pharm achieved an almost one-to-one ratio. Nine CVS were submitted and five placements were made. All of our contractors went on to complete their entire assignments with no terminations. i-Pharm's communication with both the client and the contractors was excellent throughout, which led to repeat business from the client.

We decided to hire contractors on the advise of i-Pharm and, as a result we were able to strategically delver our trials in Europe more quickly and effectively.







